Hume-Rothery was a metallurgical researcher at Oxford University. And he introduced a theory to describe the solubility of two solids. Moreover, it has two forms describing substitutional solid solutions and interstitial solid solutions. Today, these rules are often referred to as Hume Rothery Rules.
The Hume-Rothery rules are a set of rules that can predict the solubility of two solids when replacing one component with another component. In simple words, the solids must be fulfilled Hume-Rothery’s rules to make a good solid solution.
In engineering, the Hume-Rothery rules are used to predict the ability to make alloys, by mixing different solids. Before mixing the metals at experimental conditions, it’s better if we can check. For that, we can use the Hume-Rothery rules to predict the solubility of the solids. If the rules are fulfilled, there is a possibility that it makes a good solid solution.
You know that we can predict some characteristics of alloys before making them. Thus, it’s helpful to find out which combination will be stronger. And which combination will be weaker.
The Hume-Rothery rules have two forms. And one form of rules applies to substitutional solid solutions. While the other form applies to interstitial solid solutions.
Solid substitutional solution
A solid substitutional solution is a solid solution, in which the solute’s atoms replace or displace the solvent atoms in its lattice structure. At the lattice locations where the solvent atoms would generally lie, the atoms from the solute take over the atoms from the solvent.
Substitutional solid solutions are made up of two types of atoms. and one of these atoms is going to take the place of the other. Furthermore, the atoms entering must have the same atomic radius, valence, and crystal structure as the atoms in the solvent.
Interstitial solid solution
The solute atoms fill in the gaps between the solvent atoms’ crystal structures. When atoms go through interstitial zones, the bonds between them are twisted and squeezed.
Interstitial solid solutions are formed when the atoms in the solute fill the interstitial gap in the crystal structure of the solvent.
Hydrogen (H), lithium (Li), boron (B), and sodium (Na) are used to create interstitial solid solutions.
Hume-Rothery rules for substitutional solid solutions
- The radius of the atomic atoms of the solvent should not be more than 15% from the atomic radius of the solute. The crystal structures must be identical.
- The valency of the solute and the solvent must be similar.
- The electronegativity for the solute and solution must be identical.
Hume-Rothery rules for interstitial solid solutions
- The radius of the solvent atoms must be less than 59% of the radius of solvent atoms.
- The electronegativity for the solute and solution must be identical.
- The valency of the solute and the solvent must be the same.
Hume-Rothery rules are broken in some cases. For example, Interstitial atoms are smaller than the atoms of solute. So, the solubility of interstitial atoms is limited, and the first rule invalidates.
Hume-Rothery’s First Rule
The radius of the atomic atoms of the solvent should not be more than 15% from the atomic radius of the solute.
To form a solid solution, the atomic size of the solvent and solute matters. If the atoms in solute are too small compared to atoms in the solvent, it won’t make a good solution. In Hume-Rotherys’ first law, there is a specified experimented size ratio which makes an excellent solid solution.
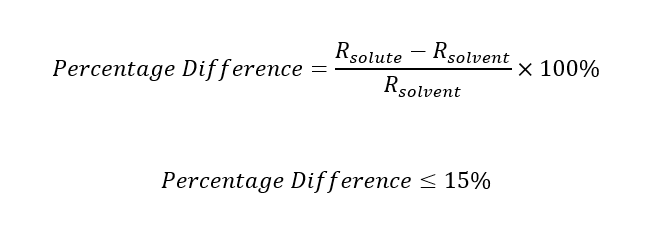
A good solid solution occurs when the relative percentage difference is less than 15%. And it must be less than 8% in order to make fully soluble (fully dissolved) solid solutions
Hume-Rothery’s Second Rule
The crystal structures must be identical.
The crystal structure of these two elements needs to be identical to have good solubility in solids.
BCC – BCC Solid Solutions
BCC means Body-Centered Cubic structure. And solid solutions can occur between two metals with BCC structure.
For example, in Tungsten alloy steel, Ferrous (Fe) is the solvent, and Tungsten (W) is the solute. Plus, both the Ferrous and the Tungsten have an FCC structure. As a result of that, a solid solution has formed.
FCC – FCC Solid Solutions
FCC means Face centered Cubic structure. And solid solutions can occur between two metals with FCC structure.
For example, Pt-Ag alloy is formed after separately heating solids to high temperatures. And the formation is only possible if the Ag/Pt molar ratio is less than 2.5 and both Platinum and Silver have the same crystal structure. Since Platinum and Silver have an FCC structure, forming Ag-Pt alloy has become possible.
Note: If the materials don’t possess the exact crystal structures, it won’t form good solutions. So, what we can expect is a shift from one phase to the other phase with a different crystal structure.
Hume-Rothery’s Third Rule
The valency of the solute and the solvent must be similar.
Metals with similar valency values dissolve more efficiently. Maximum solubility happens when the solvent and solvent atoms have identical valence. Otherwise, the electron valence difference can cause the creation of metal complexes, instead of solutions.
Hume-Rothery’s Fourth Rule
The electronegativity for the solute and solution must be identical.
Maximum solubility happens when the electronegativity gap is less than zero. So, the electronegativity of each solid must be identical to get the maximum solubility. But it can still have close values and have a good solubility.
What if the gap is large? If the gap is large, there is a greater chance to form an intermetallic complex instead of developing a solid solution.
Key Points
- Hume Rothery’s rules predict the ability to form a solid solution.
- A saturated solution is one in which the solvent dissolves only some portion of the solute. While an unsaturated solution is one that has the ability to dissolve more of the solute. Moreover, an unsaturated solution has not yet come to its saturated state.
- For substitutional solid solutions, the atomic size of the solute should be less than 15% of the solvent.
- As per Hume Rothery’s rules for an interstitial solid solution, the radius of the solute atoms should be no more than 59% of the solvent. Furthermore, they must also share similar electronegativities and valencies.
- Solvent and solute must have similar electronegativities. If the solvent and solute have a significant difference in electronegativities, they form intermetallic compounds. And not solid solutions.
- The term “random substitutional” refers to a solution where the atoms of the solvent and solvent are distributed in a random manner across the crystal’s lattice.
FAQ
Do Hume−Rothery rules apply to Nano Particles?
No, Hume−Rothery rules do not work for nanometer-sized particles. According to a research paper published by Japanese scientists they state that Hume−Rothery rules didn’t obey with their experiment with sub-nanometer-sized particles.
Do Hume Rothery rules apply to ceramics?
Yes, ceramic structures obey the rules of Hume-Rothery. For example, MgO and NiO are ceramics and the atomic size of MgO and NiO are similar, so they’re soluble.
References
- Atomic packing and size effect on the Hume-Rothery rule
- Breakdown of the Hume−Rothery Rules in Sub-Nanometer-Sized Ta-Containing Bimetallic Small Clusters
- The Hume-Rothery rules for Structurally Complex Alloy Phases



